Quality&Compliance
High quality embodied in our exceptional service is always the first priority at Guiding-Bio. A stable and sustainable manufacturing system is well guaranteed, and the transparency, traceability and reliability characterize our services along the entire supply chain.
Know well about cGMP compliance and ICH guideline and expertise in quality management and practice.
A well-trained QC team, either operators or technologists working in different positions, make sure the production run in alignment with all prescribed procedures.
A well-equipped QC lab includes HPLC, GC, IC, GPC, FTIR, UV, NMR, XRD etc
A professional QM group who is obligated to QMS Design-Daily Operation-Improvement- Compliance through gap analysis tools and systematic approach protocols etc.
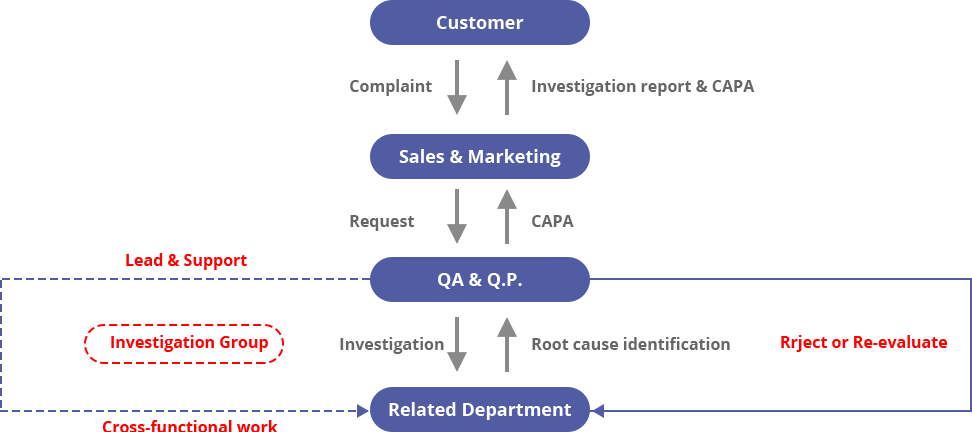
Customer Complaint Standard Procedure
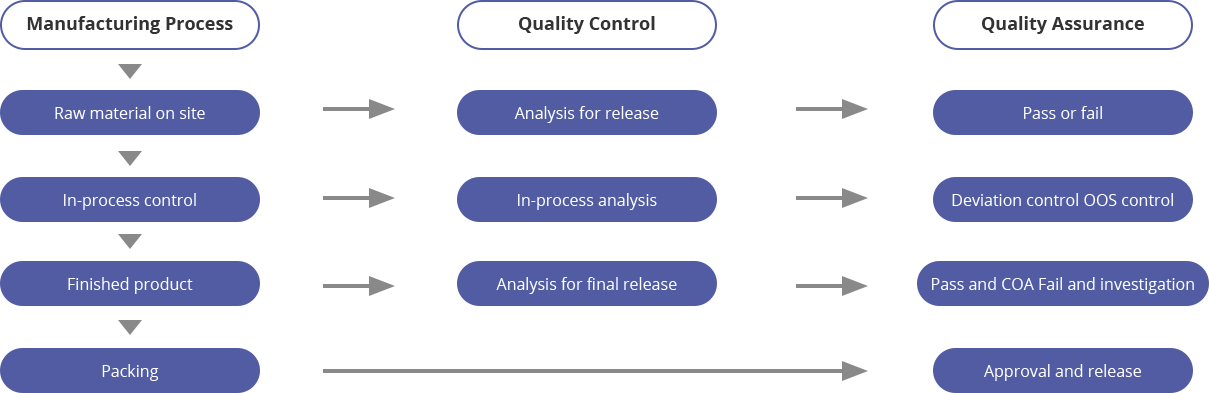
Quality Management Standard Procedure (ISO 9001:2016)
Technical & RA support & Compliance improvement
Support on technical package and registration dossiers
Full analytical support including LC/LC-MS, GC/GC-MS and NMR etc.
IP non-infringement support and the compliance program under technical confidential agreement, quality agreement and supply agreement.
In-law & In-time logistics approaches
Dedicated logistics team with a wealth of experience
Qualified third-party partners
Fully flexible supply chain model with a comprehensive logistics service
High safety standard in line with national/international laws
From scientific development over commercial manufacturing to regulatory support.
If you are interested in our products, or have any enquiry, please feel free to contact us.
About Us
Guiding-Bio is involved in screening, research and development, manufacturing of novel chemical compounds.
Quick Links
HomePhone
+86 519 8668 6860
Location
Building D, Hi-tech&Innovation Zone, No.9 East of Taihu Road, Changzhou, China
